Hi, I'm Leo Shunyakov, Medical Oncologist and Hematologist with the Central Care Cancer Center in Bolivar, MO.
In this video I'm going to discuss COSELA as a treatment option to reduce the incidence of chemo-induced myelosuppression for patients being treated with chemotherapy for extensive-stage small cell lung cancer, and what I’ve experienced with patients in my own practice.
COSELA is indicated to decrease the incidence of chemotherapy-induced myelosuppression in adult patients when administered prior to a platinum/etoposide-containing regimen or topotecan-containing regimen for ES-SCLC.
Traditional reactive therapies for treating chemo-induced myelosuppression come with their own risk and side effects, which I have seen in some of my own patients.
Since COSELA was approved, I've administered it to several of my extensive-stage small cell lung cancer patients and we're starting to use it more widely throughout the practice.
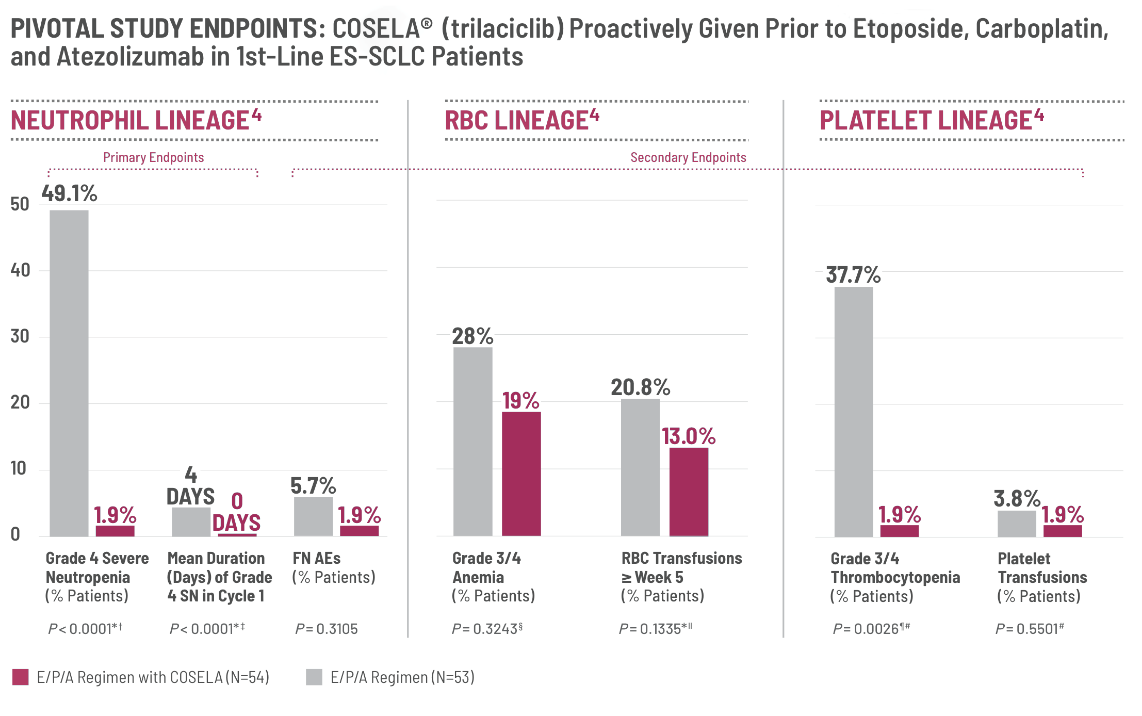
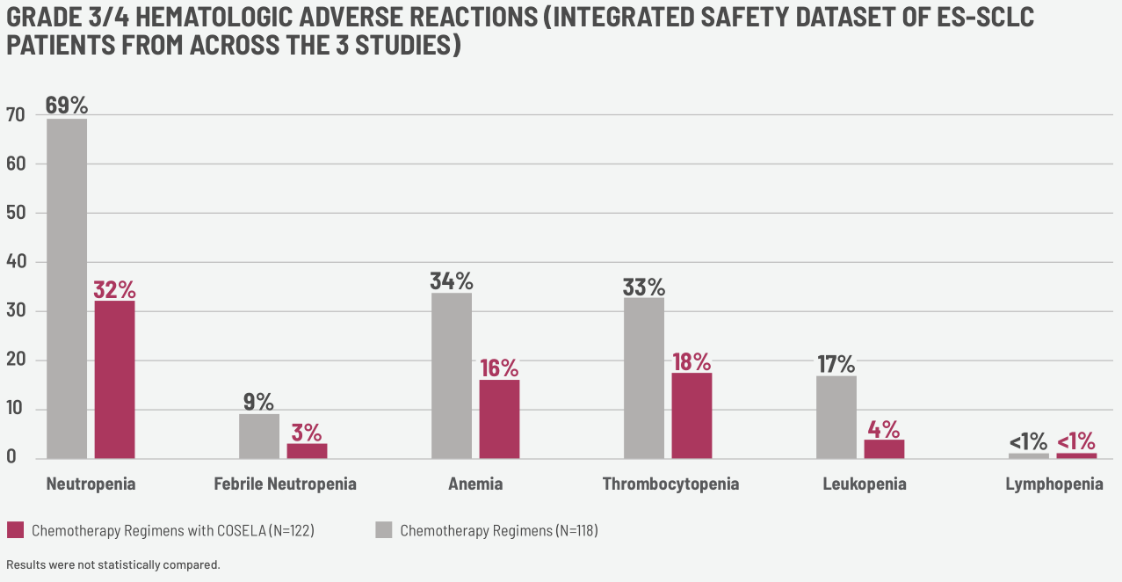
The data showing the reduction in Grade 4 severe neutropenia in the pivotal study was significant and of interest to me. This primary endpoint of the study showed 49.1% of those not receiving COSELA with grade 4 severe neutropenia and only 1.9% for patients that received COSELA.
Before I considered COSELA as a proactive treatment option for all of my appropriate extensive-stage small cell lung cancer patients, I looked at the other multilineage efficacy data, shown in this graphic, which was really impactful.
After reviewing the efficacy and safety,
I looked at the patient-reported outcomes, which were evaluated in a retrospective pooled analysis of three clinical studies to measure COSELA's effects on the patient experience.
Upon the FDA approval of COSELA, our initial focus was on the efficacy and safety data. Now, my perspective of COSELA has expanded to include the patient reported outcomes data, an important measure I consider in my evaluation of new agents.
The perspective of my patients with extensive-stage small cell lung cancer during their experience being treated with chemotherapy is of interest to me. I’m always taking their experience into consideration, and how treatments may impact that.
So, looking at these data has been helpful to me in seeing the overall patient experience on COSELA.
I also wanted to factor in COSELA’s mechanism of action and the role it may play in my patients’ treatment regimen.
When given prior to chemotherapy, COSELA essentially puts the hematopoietic stem cell progenitor cells, or HSPCs, to sleep in the G1 phase of the cell cycle.
HSPCs are typically vulnerable to chemotherapy, resulting in the depletion of multiple blood cell lineages. This can lead to certain side effects like neutropenia, anemia, and thrombocytopenia.
COSELA proactively helps protect HSPCs to enable myeloprotection. And when given prior to chemotherapy, COSELA transiently affects CDK 4 and 6 activity. This makes HSPCs less vulnerable to chemotherapy, which then in turn may help decrease hematologic side effects from the chemotherapy, such as thrombocytopenia, neutropenia, and anemia. The HSPCs can then resume their role in maintaining bone marrow function and cell production.
These multilineage data on the potential reduction from chemo-induced myelosuppression, in addition to the data provided on patient-reported outcomes, have been of great importance in my decision to use COSELA.
In my experience, all of my appropriately-treated extensive-stage small cell lung cancer patients with pre-existing conditions benefit from the use of COSELA prior to their chemotherapy regimen.
In fact, with COSELA, I’ve been able to administer full doses of chemotherapy while at the same time reducing some of the harmful chemo-induced myelosuppressive consequences. It’s worth noting that you can still use other supportive care treatments in conjunction with COSELA if needed.
With COSELA, I’ve been able to change my approach to the standard of care for my patients with extensive-stage small cell lung cancer.
I consider COSELA as an option and I’ve seen in my practice that it can be helpful for all adult patients who may experience myelosuppression. Patients without comorbidities can still develop anemia, neutropenia, or thrombocytopenia, so it’s really worth considering for all appropriate patient types.
Thank you for taking the time to watch this video. Please stay tuned for the Important Safety Information to follow. To find out more about COSELA, please visit COSELA.com.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATION
- COSELA is contraindicated in patients with a history of serious hypersensitivity reactions to trilaciclib.
WARNINGS AND PRECAUTIONS
Injection-Site Reactions, Including Phlebitis And Thrombophlebitis
- COSELA administration can cause injection-site reactions, including phlebitis and thrombophlebitis, which occurred in 56 (21%) of 272 patients receiving COSELA in clinical trials, including Grade 2 (10%) and Grade 3 (0.4%) adverse reactions. Monitor patients for signs and symptoms of injection-site reactions, including infusion-site pain and erythema during infusion. For mild (Grade 1) to moderate (Grade 2) injection-site reactions, flush line or cannula with at least 20 mL of sterile 0.9% Sodium Chloride Injection, USP or 5% Dextrose Injection, USP after end of infusion. For severe (Grade 3) or life-threatening (Grade 4) injection-site reactions, stop infusion and permanently discontinue COSELA. Injection-site reactions led to discontinuation of treatment in 3 (1%) of the 272 patients.
Acute Drug Hypersensitivity Reactions
- COSELA administration can cause acute drug hypersensitivity reactions, which occurred in 16 (6%) of 272 patients receiving COSELA in clinical trials, including Grade 2 reactions (2%). Monitor patients for signs and symptoms of acute drug hypersensitivity reactions. For moderate (Grade 2) acute drug hypersensitivity reactions, stop infusion and hold COSELA until the adverse reaction recovers to Grade ≤1. For severe (Grade 3) or life-threatening (Grade 4) acute drug hypersensitivity reactions, stop infusion and permanently discontinue COSELA.
Interstitial Lung Disease/Pneumonitis
- Severe, life-threatening, or fatal interstitial lung disease (ILD) and/or pneumonitis can occur in patients treated with cyclin-dependent kinases (CDK)4/6 inhibitors, including COSELA, with which it occurred in 1 (0.4%) of 272 patients receiving COSELA in clinical trials. Monitor patients for pulmonary symptoms of ILD/pneumonitis. For recurrent moderate (Grade 2) ILD/pneumonitis, and severe (Grade 3) or life-threatening (Grade 4) ILD/pneumonitis, permanently discontinue COSELA.
Embryo-Fetal Toxicity
- Based on its mechanism of action, COSELA can cause fetal harm when administered to a pregnant woman. Females of reproductive potential should use an effective method of contraception during treatment with COSELA and for at least 3 weeks after the final dose.
ADVERSE REACTIONS
- Serious adverse reactions occurred in 30% of patients receiving COSELA. Serious adverse reactions reported in >3% of patients who received COSELA included respiratory failure, hemorrhage, and thrombosis.
- Fatal adverse reactions were observed in 5% of patients receiving COSELA. Fatal adverse reactions for patients receiving COSELA included pneumonia (2%), respiratory failure (2%), acute respiratory failure (<1%), hemoptysis (<1%), and cerebrovascular accident (<1%).
- Permanent discontinuation due to an adverse reaction occurred in 9% of patients who received COSELA. Adverse reactions leading to permanent discontinuation of any study treatment for patients receiving COSELA included pneumonia (2%), asthenia (2%), injection-site reaction, thrombocytopenia, cerebrovascular accident, ischemic stroke, infusion-related reaction, respiratory failure, and myositis (<1% each).
- Infusion interruptions due to an adverse reaction occurred in 4.1% of patients who received COSELA.
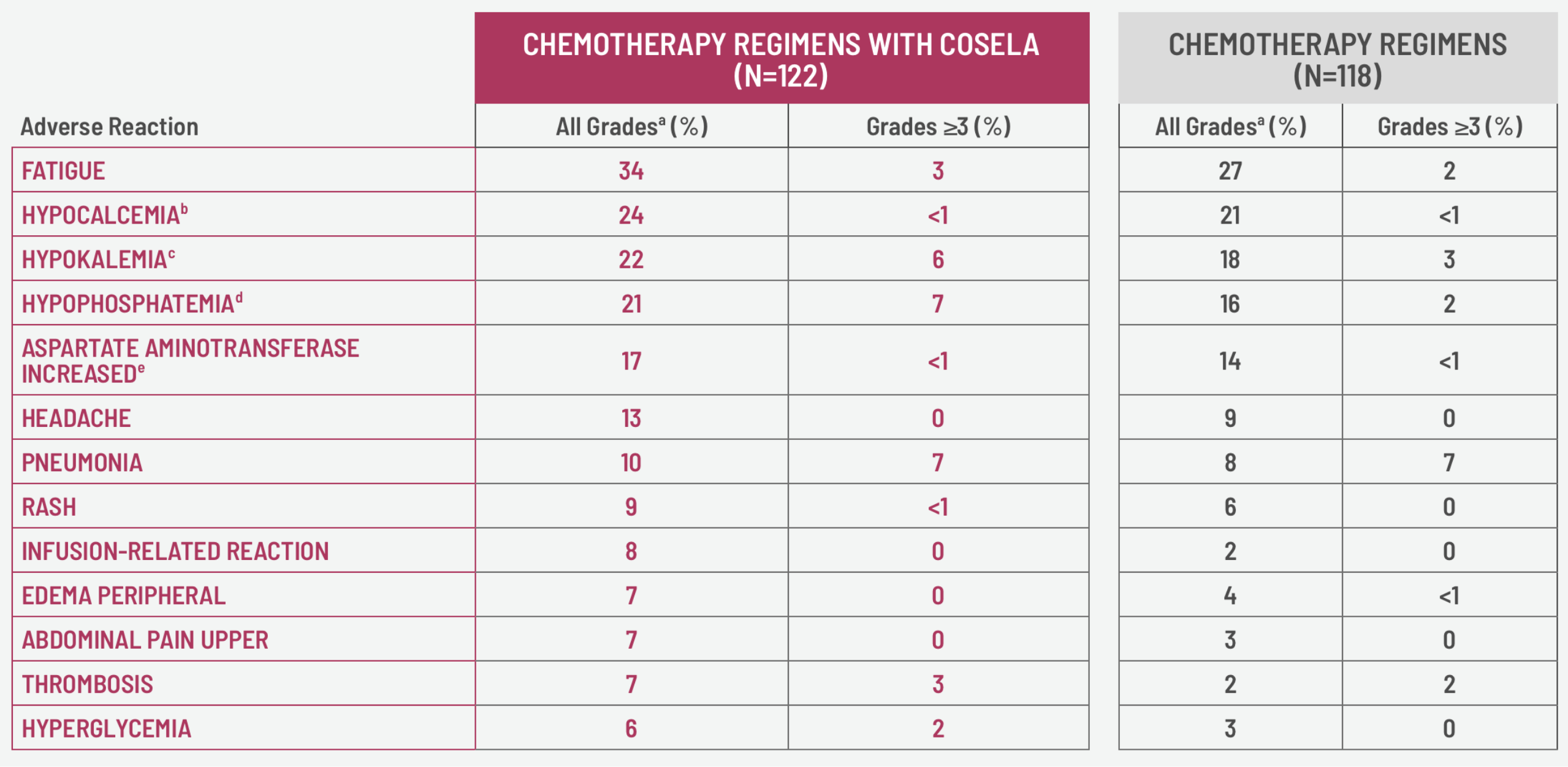
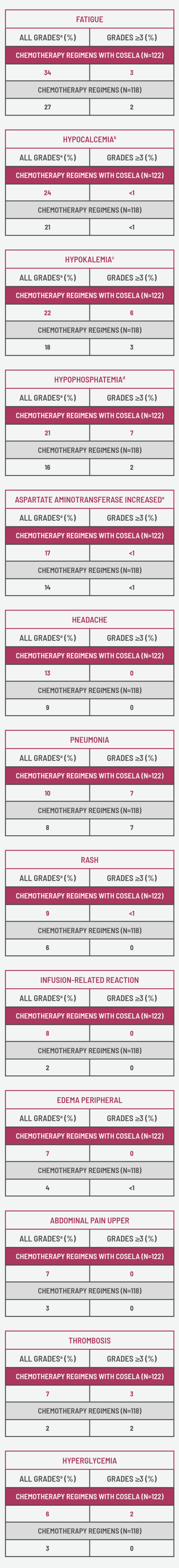
- The most common adverse reactions (≥10%) were fatigue, hypocalcemia, hypokalemia, hypophosphatemia, aspartate aminotransferase increased, headache, and pneumonia.
DRUG INTERACTIONS
- COSELA is an inhibitor of OCT2, MATE1, and MATE-2K. Co-administration of COSELA may increase the concentration or net accumulation of OCT2, MATE1, and MATE-2K substrates in the kidney (e.g., dofetilide, dalfampridine, and cisplatin).
To report suspected adverse reactions, contact G1 Therapeutics at 1-800-790-G1TX or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
This information is not comprehensive. Please see the full Prescribing Information at COSELA.com.